A conformational sensor based on genetic code expansion reveals an autocatalytic component in EGFR activation
Baumdick M, Gelléri M, Uttamapinant C, Beránek V, Chin JW, Bastiaens PIH (2018). Nat Commun
doi: 10.1038/s41467-018-06299-7.
The Bastiaens lab and the Chin lab developed a Förster resonance energy transfer (FRET)-based conformational EGFR sensor using genetic code expansion. Applying the sensor the group found a catalytic active conformation in EGFR monomers induced by phosphorylation of Y845.
This conformational transition depends on EGFR kinase activity and auto-phosphorylation on its C-termninal tail and leads to autocatalytic amplification of EGFR phosphorylation at low EGF dose.
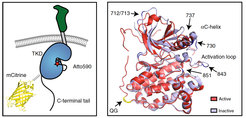
Sensor design. Left: Schematic representation of CONEGI. mCitrine is fused to the C-terminal end of the tyrosine kinase domain (TKD) using a coiled-coil linker (dashed line) and Atto590 (red star) is site-specifically attached to the activation loop. Right: Alignment of active (red; PDB: 2J5F) and inactive (cyan; PDB: 2GS7) crystal structures of the EGFR TKD. mCitrine insertion (QG, yellow) and BCNK incorporation sites (blue, black arrows) are indicated.
