Structure of a Tc holotoxin pore provides insights into the translocation mechanism
Roderer D, Hofnagel O, Benz R, Raunser S (2019). PNAS
doi: 10.1073/pnas.1909821116.
The Raunser Group together with Roland Benz from Jacobs University report 2 high-resolution cryo-EM structures of a Tc holotoxin embedded in the membrane.
The results contribute to our understanding of key steps of the prepore-to-pore transition, membrane insertion, and toxin translocation mechanisms of this biotechnologically relevant type of bacterial toxin. The study provides important insights into the mechanism of action of Tc toxins and can serve as a strong foundation for the development of biopesticides.
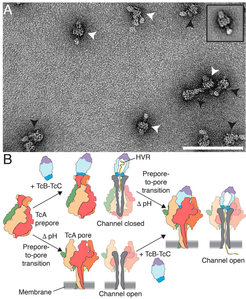
Model of 2 pathways of formation of the holotoxin pore. (A) Negative-stain electron micrograph of Tc holotoxin in nanodiscs. (B) Cartoon representation of Tc holotoxin assembly, target cell interaction, and pore formation. The first pathway shows holotoxin formation with subnanomolar affinity from pentameric TcA and the TcB-TcC fusion protein, followed by membrane association and pH-induced prepore-to-pore transition. The second pathway shows membrane association and prepore-to-pore transition of TcA, followed by holotoxin formation of the TcA pore and TcB-TcC. Subsequently, the toxic enzyme is translocated to the target cells with its C terminus first.
