Combination of pseudo-natural product design and formal natural product ring distortion yields stereochemically and biologically diverse pseudo-sesquiterpenoid alkaloids
Liu J, Flegel J, Otte F, Pahl A, Sievers S, Strohmann C, Waldmann H (2021). Angew Chem Int Ed Engl.
The Waldmann group describes the synthesis and biological evaluation of a new natural product-inspired compound class obtained by combination of the conceptually complementary pseudo-natural product (pseudo-NP) design strategy and a formal adaptation of the complexity-to-diversity ring distortion approach.
Fragment-sized α-methylene-sesquiterpene lactones (SLs) were combined with alkaloid-derived pyrrolidine fragments by means of highly selective stereocomplementary 1,3-dipolar cycloaddition Reactions.
Biological investigation of the compound collection led to the discovery of a novel chemotype inhibiting Hedgehog-dependent osteoblast differentiation.
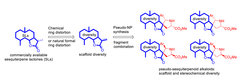
Combination of the conceptually complementary formal ring distortion- and pseudo-natural product strategies yields novel natural product-inspired bioactive pseudo-sesquiterpenoid alkaloids.
