Exploiting π and Chalcogen Interactions for the β-Selective Glycosylation of Indoles through Glycal Conformational Distortion
Guo H, Kirchhoff JL, Strohmann C, Grabe B, Loh CCJ (2023). Angew Chem Int Ed Engl
Source
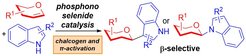
The Loh research group disclose recently a robust and β-selective phosphonoselenide catalyzed 2-deoxyglycosylation of indoles with glycals. The strategy is amenable to both the C3 and N1 positions of indoles and hence opened up a broad access platform towards diverse β-indolyl glycosides. Such scaffolds are embedded in numerous bioactive molecular scaffolds that with anti-inflammatory, anti-diabetic and neuro-protective activity.
Combining insights gathered from mechanistic investigations and computations, the Loh group demonstrated that the aromatic and chalcogenic fragments of the catalyst can be synergistically harnessed for glycal conformational distortions, through a myriad of π-π, CH-π as well as Se···O interactions on the α-face. This enforces a boat conformation which exposes the convex β-face for nucleophilic attack. Kinetic studies and deuterated control experiments suggested a step-wise mechanism whereby the glycosyl donor, acceptor and the catalyst are pivotally involved in the rate limiting step.