Clathrin's adaptor interaction sites are repurposed to stabilize microtubules during mitosis
Rondelet A, Lin YC, Singh D, Porfetye AT, Thakur HC, Hecker A, Brinkert P, Schmidt N, Bendre S, Müller F, Mazul L, Widlund PO, Bange T, Hiller M, Vetter IR, Bird AW (2020). J Cell Biol
doi: 10.1083/jcb.201907083.
The Bird group together with the Vetter Group and Michael Hiller from the Max Planck Institute of Molecular Cell Biology and Genetics in Dresden have unravelled how clathrin stabilizes microtubules to ensure mitotic spindle stability and efficient chromosome alignment. This function of clathrin is mediated by the recruitment of the microtubule stabilizing protein GTSE1 via adaptor interaction sites on clathrin heavy chains (CHC). Structural analysis reveal that these sites interact directly with clathrin-box motifs on GTSE1. Disruption of the Clathrin-GTSE1 interaction releases GTSE1 from spindles thereby causing defects in chromosome alignment.
GTSE1 promotes microtubule stability by inhibiting the activity of the microtubule-destabilizing enzyme MCAK, as shown by Bird et al. in JCB in 2016. Thus, a major mechanism by which clathrin stabilizes microtubules is ultimately inhibition of MCAK.
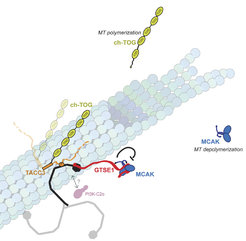
Model of the clathrin/TACC3 complex on a MT. Schematic model of the clathrin/TACC3 complex on a MT based on studies to date. The Aurora A–dependent phosphorylation of S558 on TACC3 is represented as a P. TACC3 is also bound to the MT polymerase ch-TOG. The CHC NTD recruits GTSE1 to inhibit the MT depolymerase MCAK. It is unknown whether PI3KC2a and GTSE1 can interact with a single CHC on the spindle. Relative protein sizes are roughly conserved
