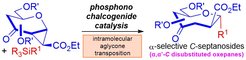
Stereoselective Entry into α,α’-C-Oxepane Scaffolds through a Chalcogen Bonding Catalyzed Strain-Release C-Septanosylation Strategy
Ma W, Schmidt A, Strohmann C, Loh CCJ. (2024). Angew Chem Int Ed Engl.
Through the exploitation of chalcogen bonding catalysis, the Loh group lately reported a rare thermodynamically favored strain-release C-glycosylation approach to access the synthetically challenging, but yet biologically relevant α,α’-C-disubstituted oxepane scaffold. Besides tolerating a broad range of silylated C-nucleophiles, this strategy taps upon an unknown intramolecular aglycone transposition pathway via a pentavalent silcon intermediate to achieve the excellent stereoselectivity outcome.