Structural basis of TRPC4 regulation by calmodulin and pharmacological agents
Vinayagam D, Quentin D, Yu-Strzelczyk J, Sitsel O, Merino F, Stabrin M, Hofnagel O, Yu M, Ledeboer MW, Nagel G, Malojcic G, Raunser S (2020) eLife
Quelle
Canonical transient receptor potential channels (TRPC) are involved in receptor-operated and/or store-operated Ca2+ signaling. Inhibition of TRPCs by small molecules was shown to be promising in treating renal diseases. In cells, the channels are regulated by calmodulin. Molecular details of both calmodulin and drug binding have remained elusive so far. Here we report structures of TRPC4 in complex with three pyridazinone-based inhibitors and calmodulin. The structures reveal that all the inhibitors bind to the same cavity of the voltage-sensing-like domain and allow us to describe how structural changes from the ligand binding site can be transmitted to the central ion-conducting pore of TRPC4. Calmodulin binds to the rib helix of TRPC4, which results in the ordering of a previously disordered region, fixing the channel in its closed conformation. This represents a novel calmodulin-induced regulatory mechanism of canonical TRP channels.
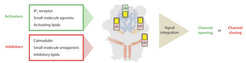
Individual or simultaneous binding of activators and/or inhibitors modulate the channel gating. Interestingly, modulation sites, i.e. ligand pockets or structural features to which certain compounds or regulatory proteins bind, can accommodate both activators and inhibitors. Thus, these regions can be considered as activity switches. Binding of activators results in an “ON” position, whereas inhibitor binding causes an “OFF” state. In the case that multiple modulators bind simultaneously, all signals are integrated to determine whether the channel opens or remains closed.
