Morphological profiling identifies the motor protein Eg5 as cellular target of spirooxindoles
Liu J, Mallick S, Xie Y, Grassin C, Lucas B, Schölermann B, Pahl A, Scheel R, Strohmann C, Protzel C, Berg T, Merten C, Ziegler S, Waldmann H. Angew Chem Int Ed Engl. (2023)
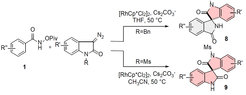
Oxindoles and iso-oxindoles are natural product-derived scaffolds that provide inspiration for the design and synthesis of novel biologically relevant compound classes. Notably, the spirocyclic connection of oxindoles with iso-oxindoles has not been explored by nature but promises to provide structurally related bioactive compounds endowed with novel bioactivity. Therefore, methods for their efficient synthesis and the conclusive discovery of their cellular targets are highly desirable. We describe a selective Rh(III)-catalyzed scaffold-divergent synthesis of spirooxindole-isooxindoles and spirooxindole-oxindoles from differently protected diazooxindoles and N-pivaloyloxy aryl amides which includes a functional group-controlled Lossen rearrangement as key step. Unbiased morphological profiling of a corresponding compound collection in the Cell Painting assay efficiently identified the mitotic kinesin Eg5 as the cellular target of the spirooxindoles, which defines a unique Eg5 inhibitor chemotype.