Structure of the Mon1-Ccz1 complex reveals molecular basis of membrane binding for Rab7 activation
Klink BU, Herrmann E, Antoni C, Langemeyer L, Kiontke S, Gatsogiannis C, Ungermann C, Raunser S, Kümmel D (2022). Proc Natl Acad Sci U S A
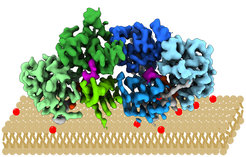
Here, the Raunser Group present a high-resolution cryogenic electron microscopy structure of the Mon1-Ccz1 complex that reveals its architecture in atomic detail. Mon1 and Ccz1 are arranged side by side in a pseudo-twofold symmetrical heterodimer. The three Longin domains of each Mon1 and Ccz1 are triangularly arranged, providing a strong scaffold for the catalytic center of the GEF. At the opposite side of the Ypt7-binding site, a positively charged and relatively flat patch stretches the Longin domains 2/3 of Mon1 and functions as a phosphatidylinositol phosphate-binding site, explaining how the GEF is targeted to membranes. Our work provides molecular insight into the mechanisms of endosomal Rab activation and serves as a blueprint for understanding the function of members of the Tri Longin domain Rab-GEF family.