Principle and design of pseudo-natural products
Karageorgis G, Foley DJ, Laraia L and Waldmann H (2020) Nat Chem
doi: 10.1038/s41557-019-0411-x
Evolution has developed a plethora of highly effective naturla products (NPs) that fulfill essential tasks in both eukaryotes and prokaryotes. Their mode of action is mainly based on structural properties that allow an effective binding of target proteins resulting in the modulation of their activity. These properties have been selected and evolved by nature to near-perfection, making natural products a significant source of inspiration towards the discovery of new bioactive compounds based on novel molecular scaffolds.
However, only a small number of available guiding synthetic strategies limits both the number and types of compounds accesible.
The group of Prof. Dr. Dr. h.c. Herbert Waldmann has developed new design and synthesis principles to go beyond the chemical space explored by nature by combining the principles of biology-oriented synthesis (BIOS) and fragment-based compound design. In simple terms, scaffolds from different NPs are fragmented and reconnected into new alternative molecular frameworks, so called pseudo natural produts.
In their latest articel "principle and design of pseudo-natural products" Herbert Waldmann and his colleagues identify design principles and connectivity patterns that would provide access to unprecedented pseudo-NP classes.
(1) In general, employed fragments should derive from NPs with diverse bioactivities. (2) They should be biosynthetically unrelated to combine different structural parameters for binding to proteins. (3) To ensure structural diversity the fragments should contain complementary heteroatoms. Since stereogenic content correlates with bioactivity, the fragments should also be combined into a three-dimensional scaffold amenable to furhter derivatization. (4) NP fragemnts derived from NPS with diverse bioactivities should be combined to maximize the biological relevance of the resulting pseudo-NP.
Read more on pseudo-natural prodcuts in the newsroom: Nature 2.0 – Inspiring new drug discovery by pseudo natural products
However, only a small number of available guiding synthetic strategies limits both the number and types of compounds accesible.
The group of Prof. Dr. Dr. h.c. Herbert Waldmann has developed new design and synthesis principles to go beyond the chemical space explored by nature by combining the principles of biology-oriented synthesis (BIOS) and fragment-based compound design. In simple terms, scaffolds from different NPs are fragmented and reconnected into new alternative molecular frameworks, so called pseudo natural produts.
In their latest articel "principle and design of pseudo-natural products" Herbert Waldmann and his colleagues identify design principles and connectivity patterns that would provide access to unprecedented pseudo-NP classes.
(1) In general, employed fragments should derive from NPs with diverse bioactivities. (2) They should be biosynthetically unrelated to combine different structural parameters for binding to proteins. (3) To ensure structural diversity the fragments should contain complementary heteroatoms. Since stereogenic content correlates with bioactivity, the fragments should also be combined into a three-dimensional scaffold amenable to furhter derivatization. (4) NP fragemnts derived from NPS with diverse bioactivities should be combined to maximize the biological relevance of the resulting pseudo-NP.
Read more on pseudo-natural prodcuts in the newsroom: Nature 2.0 – Inspiring new drug discovery by pseudo natural products
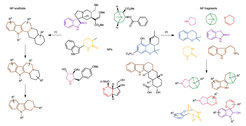
BIOS and pseudo-NPs are both based on NP structures. Scaffold synthesis and decoration following the principle of BIOS leads to NP-inspired compounds (1). Design of pseudo-NP collections by de novo combinationof NP fragments leads to NP-inspired compounds (2). Different colours highlight different NP fragments, or substructures depending on the strategy followed. β-GluO, β-glucosyl.
