Glycan-dependent Cell Adhesion Mechanism of Tc Toxins
Roderer D, Bröcker F, Sitsel O, Kaplonek P, Leidreiter F, Seeberger PH, Raunser S (2020) Nat Commun
doi: 10.1038/s41467-020-16536-7.
The Raunser Lab together with the Seeberger lab from MPI Potsdam provide new insights into the binding of Tc-Toxins to host receptors. They identify heparins/heparan sulfates and Lewis antigens as receptorsfor different TcAs from insect and human pathogens. Glycan array screening reveals that all tested TcAs bind negatively charged heparins.
Cryo-EM structures of Morganella morganii TcdA4 and Xenorhabdus nematophila XptA1 reveal that heparins/heparan sulfates unexpectedly bind to different regions of the shell domain, including receptor-binding domains. In addition, Photorhabdus luminescens TcdA1 binds to Lewis antigens with micromolar affinity. Here, the glycan interacts with the receptor-binding domain D of the toxin.
The results suggest a glycan dependent association mechanism of Tc toxins on the host cell surface.
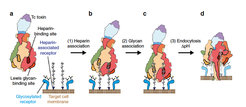
Model of the intoxication mechanism of Tc. a Tc toxin and target cells with multiple glycosylated and heparin-associated receptors. The binding sites for heparin and Lewis antigens are indicted on two protomers. b Tc toxin associates to heparin-associated receptors on target cells. c In the second association reaction, Tc toxin binds to additional glycosylated receptors, resulting in a tighter toxin-cell complex. d Upon endocytosis, the shift of pH to acidic values induces prepore-to-pore transition and membrane permeation of the Tc toxin.
