The mechanism of activation of the actin binding protein EHBP1 by Rab8 family members
Rai A, Bleimling N, Vetter IR, Goody RS (2020) Nat Commun
doi: 10.1038/s41467-020-17792-3.
The group of Roger Goody unraveld the underlying mechanism of how Rab8 and Eps15-homology domain containing protein mediated vesicular trafficking is regulated by the adaptor protein EHBP1. Interestingly, both termini of EHBP1 have membrane targeting potential.
While its N-terminal C2 (purple) domain associates with the membrane directly, binding of the C-terminus is auto-inhibited by a intramolecular complex of the bMERB (green) and the CH domain (orange). Biochemical and structural analysis revealed how Rab8 frees the CH domain and allows it to interact with the actin cytoskeleton.
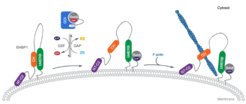
In the absence of active Rab8, EHBP1 exists in a closed conformation in which the CH domain interacts with its bMERB domain. Rab8 is recruited to the membrane by its GEF molecule (e.g., Rabin 8 or GRAB) and the resulting active GTP bound Rab8 interacts with the EHBP1 bMERB domain; this interaction makes the CH domain available for F-actin interaction.
