Processing Temporal Growth Factor Patterns by an Epidermal Growth Factor Receptor Network Dynamically Established in Space
Koseska A, Bastiaens PIH (2020) Annu Rev Cell Biol
Quelle
The proto-oncogenic epidermal growth factor (EGF) receptor (EGFR) is a tyrosine kinase whose sensitivity and response to growth factor signals that vary over time and space determine cellular behavior within a developing tissue. The molecular reorganization of the receptors on the plasma membrane and the enzyme-kinetic mechanisms of phosphorylation are key determinants that couple growth factor binding to EGFR signaling. To enable signal initiation and termination while simultaneously accounting for suppression of aberrant signaling, a coordinated coupling of EGFR kinase and protein tyrosine phosphatase activity is established through space by vesicular dynamics. The dynamical operation mode of this network enables not only time-varying growth factor sensing but also adaptation of the response depending on cellular context. By connecting spatially coupled enzymatic kinase/phosphatase processes and the corresponding dynamical systems description of the EGFR network, we elaborate on the general principles necessary for processing complex growth factor signals.
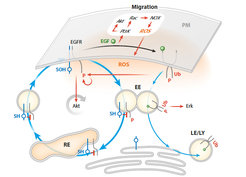
Dynamics of spatial EGFR cycles. EGFR vesicular trafficking through the endosomal system is promoted through the EGFRPI3K-Akt-dependent activation of the early endosomal effector PIKfyve, which phosphorylates PI(3)P to PI(3,5)P. This enhances the transition of EGFR from EEs to other endocytic compartments, namely LEs or REs. Ub functions as a sorting signal in the vesicular trafficking of EGFR through the endosomal system: Ligand-bound, ubiquitinated receptor complexes are unidirectionally trafficked from the plasma membrane through the EEs and LEs to be degraded in LYs, whereas the internalized, nonubiquitinated monomeric receptors are redirected from the EEs to the REs back to the plasma membrane.
