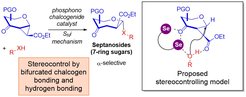
Cooperative Bifurcated Chalcogen Bonding and Hydrogen Bonding as Stereocontrolling Elements for Selective Strain-Release Septanosylation
Ma W, Kirchhoff JL, Strohmann C, Grabe B, Loh CCJ (2023). J. Am. Chem. Soc.
The Loh research group lately reported in JACS the exploitation of an unconventional bifurcated chalcogen bonding and hydrogen bonding (HB) network, which paves a robust catalytic strategy into biologically useful seven-membered ring sugars known as septanosides. In successful collaboration with the Strohmann’s group in TU Dortmund, the absolute configurations and the seven-ring architecture could be unambiguously confirmed by X-ray crystallography.
Septanosides are endowed with a biologically interesting oxepane scaffold which is hitherto difficult to gain access synthetically. They are recognized to be glycomimetics of the more commonly occurring five- or six ring sugars. Septanosides extracted from Atriplex portulacoides roots such as Portulasoid and Septanoecdysone are known to possess anti-bacterial properties as well as anticholinesterase activity, Septanosides were demonstrated as useful biological probes that can penetrate the bacterial membrane of E. coli.
A key innovation of the current concept is the combination of an emerging non-classical noncovalent interaction – chalcogen bonding interaction – with carbohydrate synthesis, so that an unprecedented ternary complex network could be harnessed to guide the α-facial delivery of the aglycone. The exclusive α-selectivity of the current method also showcases a distinctive advance over previous septanoside synthesis methods, where reliable anomeric selectivity was difficult to attain.
Through DFT modeling and kinetic studies, the data corroborated that a dissociative SNi type mechanism forms the stereocontrolling basis for the excellent α-selectivity.