Structures from intact myofibrils reveal mechanism of thin filament regulation through nebulin
Wang Z, Grange M, Pospich S, Wagner T, Kho AL, Gautel M, Raunser S (2022). Science
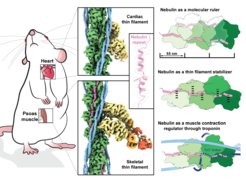
A team of researchers, led by Stefan Raunser at the MPI in Dortmund in collaboration with Mathias Gautel at King's College London, used electron cryo-tomography to decipher the structure of the muscle protein nebulin in impressive detail. This in situ reconstruction provided high-resolution details of the interaction between nebulin and actin, demonstrating the stabilizing role of nebulin. Myosin bound to the thin filaments exhibited different conformations of the neck domain, highlighting its inherent structural variability in muscle. Unexpectedly, nebulin did not interact with myosin or tropomyosin, but it did interact with a troponin T linker through two potential binding motifs on nebulin, explaining its regulatory role. Our structures support the role of nebulin as a thin filament “molecular ruler” and provide a molecular basis for studying nemaline myopathies.