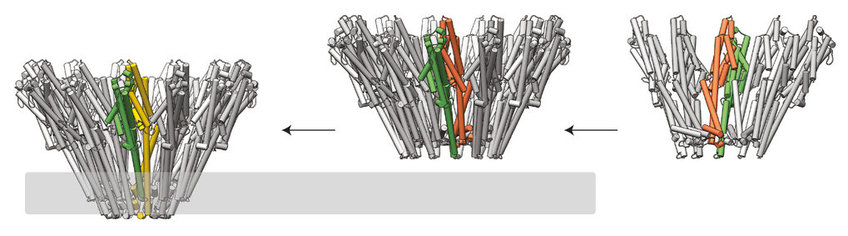
How bacteria puncture the membrane of insect cells
A variety of bacterial pathogens attack host cells by producing pore forming toxins that create unregualated pores in their membranes. The Raunser lab reports the cryo-EM structure of the XaxAB pore complex from Xenorhabdus nematophila and the crystal structures of the soluble monomers of XaxA and XaxB.
Some bacteria make toxins that punch large holes into the membranes of host cells, destroying them like a puncture destroys a football. These “pore-forming toxins” allow many bacterial species to infect a variety of organisms, from insects to humans. Some sophisticated pore-forming toxins, such as the anthrax toxin, do not only form a pore but also use it to flood lethal toxins into the cell to kill it.
One bacterium called Xenorhabdus nematophila punctures the membranes of insect cells, using the same type of pore-forming toxins that other bacteria use to infect humans. Previous research has shown that two proteins – components A and B – form these pore-forming toxins. Given this two-protein formation, some scientists predicted these pore-forming toxins might act like those of the anthrax bacterium: one component forms the pore; the other component poisons the cell. But without detailed images of this pore-forming toxin’s structure, understanding exactly how these two components work together is almost impossible.
To explore how components A and B operate within Xenorhabdus nematophila, Schubert et al. captured images of the molecular structure of the two proteins. Common methods reliant on X-rays and electron microscopes revealed the layouts of both components. By visualizing the proteins at different stages, Schubert et al. observed key structural changes that enable them to form the pore and puncture a host cell.
Component A binds to component B’s back, forming a subunit – twelve to fifteen of which then conjoin as the pore-forming toxin. Schubert et al. conclude that component A stabilizes each subunit on the membrane and activates component B, which then punctures the membrane by swinging out its lower end. Unlike the anthrax pore-forming toxin, both components collaborate to form the pore complex and puncture the membrane. These results provide a foundation of knowledge about what these toxins look like and how they operate. More research building upon this structural analysis may help scientists develop antibiotics that prevent bacteria from destroying human cells.
This text is an eLife digest