Nature 2.0 – Inspiring new drug discovery by pseudo natural products
Almost a third of the available pharmaceuticals is based on natural products (NP). The discovery of new NP-inspired drugs however, is slow due to their limited chemical diversity, their high chemical complexity and the resulting low yields. The group of Prof. Dr. Dr. h.c. Herbert Waldmann has found a way to bypass these limitations by developing substances with new molecular frameworks that don’t look like natural substances, but hold the same biological properties.
3 October 2018
Evolution has developed a plethora of highly effective NPs that fulfill essential tasks in both eukaryotes and prokaryotes. Their mode of action is mainly based on structural properties that allow an effective binding of target proteins resulting in the modulation of their activity. These properties have been selected and evolved by nature to near-perfection. The molecular scaffolds of NPs thereby provide a good starting point of biological relevance for the development of NP-inspired substances. Through Biology Oriented Synthesis (BIOS) NP scaffolds are structurally reduced to less complex scaffolds that do not lose their properties but provide a better platform for synthetic modifications. However, the same natural selection process that created the NPs also limited their number and diversity. For this reason NPs only occupy a relatively small fraction of the chemical space covered by biologically relevant substances.
The group of Prof. Dr. Dr. h.c. Herbert Waldmann has developed new design and synthesis principles to go beyond the chemical space explored by nature by combining the principles of BIOS and fragment-based compound design. In simple terms, scaffolds from different NPs were fragmented and reconnected into to new alternative molecular frameworks. To achieve a higher potential bioactivity the group applied basic guidelines based on known structural characteristics of NPs. In general, employed fragments should derive from NPs with diverse bioactivities. They should be biosynthetically unrelated to combine different structural parameters for binding to proteins. To ensure structural diversity the fragments should contain complementary heteroatoms. Since stereogenic content correlates with bioactivity, the fragments should also be combined into a three-dimensional scaffold.
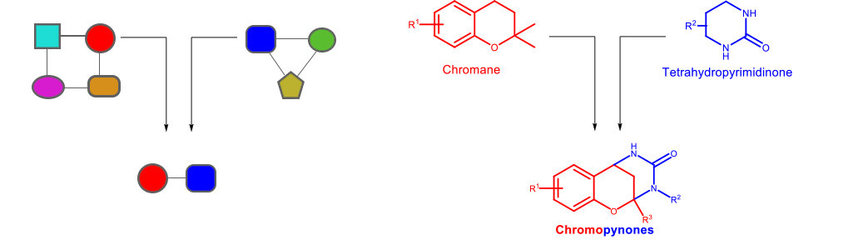
Following these principles a new class of pseudo NPs was designed, termed chromopynones as they consist of a chromane and a tetrahydropyrimidinone fragment. The oxygen-rich chromane occure widely in natural products with a variety of bioactivities. The nitrogen-containing tetrahydropyrimidine (THPM) is a core fragment of an antibiotic class. Biological investigations of the chromopynones revealed a restriction in the increased uptake of glucose in cancer cells. This effect results from inhibition of the glucose transporters GLUT-1 and -3 and leads to the suppression of cancer cell growth.
Since the chromopynones revealed a new biologically activity not related to the natural product counter parts, the chemists wondered about their chemical relationship. Atom connectivity patterns, analyzed by cheminformatics tools, lead to the seemingly odd finding that the chromopynones are not very natural product-like. However, the chromopynones are a product which is not encountered by nature. They occupy a chemical space that does not overlap with the space defined by NPs and BIOS compounds.
This pioneering strategy gives access to larger areas of the biologically relevant chemical space not covered by nature and opens the door to a new class of nature-inspired products with new biological activity. Connecting smaller synthetic fragments to afford complicated structures is a fundamental part of chemical training and doing so in alternative patterns can lead to new opportunities. This concept and the underlying design principles need to be further validated by developing different classes of pseudo NPs. The example of chromopynones as a novel inhibitor of glucose uptake in cancer cells shown here, promises to serve as an inspiration for new drug discovery programmes aimed at tumor metabolism.
JJ/GK