A barbed end interference mechanism reveals how capping protein promotes nucleation in branched actin networks
Funk J, Merino F, Schaks M, Rottner K, Raunser S, Bieling P (2021) Nature Communications
Heterodimeric capping protein (CP/CapZ) is an essential factor for the assembly of branched
actin networks, which push against cellular membranes to drive a large variety of cellular
processes. Aside from terminating filament growth, CP potentiates the nucleation of actin
filaments by the Arp2/3 complex in branched actin networks through an unclear mechanism.
Here, we combine structural biology with in vitro reconstitution to demonstrate that CP not
only terminates filament elongation, but indirectly stimulates the activity of Arp2/3 activating
nucleation promoting factors (NPFs) by preventing their association to filament barbed ends.
Key to this function is one of CP’s C-terminal “tentacle” extensions, which sterically masks
the main interaction site of the terminal actin protomer. Deletion of the β tentacle only
modestly impairs capping. However, in the context of a growing branched actin network, its
removal potently inhibits nucleation promoting factors by tethering them to capped filament
ends. End tethering of NPFs prevents their loading with actin monomers required for activation
of the Arp2/3 complex and thus strongly inhibits branched network assembly both in
cells and reconstituted motility assays. Our results mechanistically explain how CP couples
two opposed processes—capping and nucleation—in branched actin network assembly.
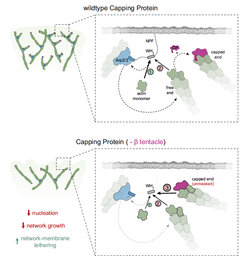
former generates the nucleation-competent NPF state (1), whereas binding of the latter sequesters the NPF in an inactive configuration (2). Binding of
wildtype capping protein masks the terminal subunits preventing NPF interaction, thereby indirectly stimulating nucleation. Bottom panel: Deletion of the β tentacle unmasks the terminal actin protomer, allowing capped ends to tether additional NPF proteins in an inactive state (3). This strengthens the
tethering between actin network and the membrane and decreases the nucleation rate and network growth velocity.
