Mechanism of centromere recruitment of the CENP-A chaperone HJURP and its implications for centromere licensing
Pan D, Walstein K, Take A, Bier D, Kaiser N, Musacchio A (2019). Nature Communications
doi: 10.1038/s41467-019-12019-6.
The Musacchio lab revelead how the CENP-A chaperone HJURP interacts with its partners and how this leads to CENP-A deposition.
Using a powerful combination of in vivo and in vitro tools thelab could show that two repeats in human HJURP proposed to be functionally distinct are in fact interchangeable and bind concomitantly to the 4:2:2 Mis18α:Mis18β:M18BP1 complex without dissociating it. HJURP binds CENP-A:H4 dimers, and therefore assembly of CENP-A:H4 tetramers must be performed by two Mis18αβ:M18BP1:HJURP complexes, or by the same complex in consecutive rounds. The Mis18α N-terminal tails blockade two identical HJURP-repeat binding sites near the Mis18αβ C-terminal helices.
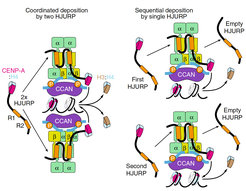
Two models of HJURP recruitment to the Mis18 receptor and the deposition of CENP-A:H4 dimers
