Optogenetic Tuning Reveals Rho Amplification-Dependent Dynamics of a Cell Contraction Signal Network
Kamps, D, Koch J, Juma VO, Campillo-Funollet E, Graessl M, Banerjee S, Mazel T, Chen X, Wu YW, Portet S, Madzvamuse A, Nalbant P, Dehmelt L (2020) Cell Reports
Source
Local cell contraction pulses play important roles in tissue and cell morphogenesis. Here, we improve a chemo-optogenetic approach and apply it to investigate the signal network that generates these pulses. We use these measurements to derive and parameterize a system of ordinary differential equations describing temporal signal network dynamics. Bifurcation analysis and numerical simulations predict a strong dependence of oscillatory system dynamics on the concentration of GEF-H1, an Lbc-type RhoGEF, which mediates the positive feedback amplification of Rho activity. This prediction is confirmed experimentally via optogenetic tuning of the effective GEF-H1 concentration in individual living cells. Numerical simulations show that pulse amplitude is most sensitive to external inputs into the myosin component at low GEF-H1 concentrations and that the spatial pulse width is dependent on GEF-H1 diffusion. Our study offers a theoretical framework to explain the emergence of local cell contraction pulses and their modulation by biochemical and mechanical signals.
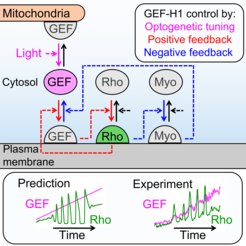
Top panels: Causal connections between GEF-H1 (GEF), Rho, and Myosin (Myo) in the cell contraction signal network. The effective, cytosolic concentration of GEF-H1 is tuned in living cells via light-controlled release from mitochondria-bound anchors. Bottom panels: Prediction and experimental confirmation of altered Rho activity dynamics (green lines) in response to gradually increasing GEF-H1 concentrations (magenta lines).
