Interplay of kinetochores and catalysts drives rapid assembly of the mitotic checkpoint complex
Sethi S, Ghetti S, Cmentowski V, Guerriere TB, Stege P, Piano V, Musacchio A (2025). Nat. Commun.
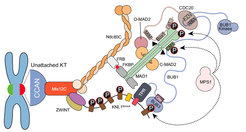
The spindle assembly checkpoint (SAC) ensures mitotic exit occurs only after sister chromatid biorientation, but how this coordination is mechanistically achieved remains unclear. Kinetochores, the megadalton complexes linking chromosomes to spindle microtubules, contribute to SAC signaling. However, whether they act solely as docking platforms or actively promote the co-orientation of SAC catalysts such as MAD1:MAD2 and BUB1:BUB3 remains unresolved. Here, we reconstitute kinetochores and SAC signaling in vitro to address this question. We engineer recombinant kinetochore particles that recruit core SAC components and trigger checkpoint signaling upon Rapamycin induction, and test their function using a panel of targeted mutants. At approximately physiological concentrations of SAC proteins, kinetochores are essential for efficient mitotic checkpoint complex (MCC) assembly, the key effector of SAC signaling. Our results suggest that kinetochores serve not only as structural hubs but also as catalytic platforms that concentrate and spatially organize SAC components to accelerate MCC formation and ensure timely checkpoint activation.