Ordered from afar: How germs’ toxins become dangerous
Max Planck Institute’s researchers highlight the mechanism that enhances toxicity of pathogens in the human cell
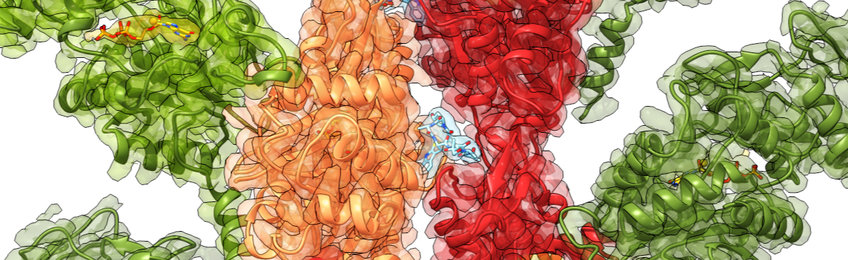
The bacterium P. aeruginosa causes the most common secondary infection in hospital patients with influenza, COVID-19 or cystic fibrosis and it is resistant to antibiotics. Another bacterial pathogen, Vibrio vulnificus, is found in raw seafood and brackish waters and can have rare, but deadly consequences for human beings. To poison the cells, both pathogens use toxins called ExoY that are almost inactive inside the bacteria. Once injected into cells, however, ExoY takes a turning point as it becomes ten thousand times more active. Yet, the exact mechanism that leads to this ten thousand fold activity was until recently unknown.
For the first time, researchers from the Max Planck Institute of Molecular Physiology in Dortmund, Germany, in collaboration with the Institut Pasteur in Paris, France, have discovered how the structure of the toxin becomes more ordered when interacting with actin, one of the main components of the cytoskeleton. It appears that the docking of ExoY to actin induces a stabilisation downhill at the toxin enzymatic core, which becomes apt to perform its venomous function. The scientists used cryo-electron microscopy (cryo-EM) and computational simulations to obtain the structural details of the toxins before and after binding, as well as enzymatic assays to quantify the activity of the toxin.
Stefan Raunser, Director at the Max Planck Institute of Molecular Physiology in Dortmund, and lead author of this study, explains the research in detail:
What is the discovery that you made and why is it exciting?
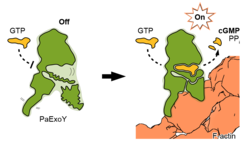
Our team has revealed, at the molecular level, how the toxin ExoY is activated inside the cell by interacting with a molecule of the host. Interestingly, this is not a direct effect. Upon being injected into the cell, the toxin docks to actin filaments, stabilizing the peripheral parts of the toxin that were twisting and bending before. This conformational change also induces a stabilisation of the active core of the toxin, which is then ready for its devastating enzymatic activity. We call this rearrangement starting from afar “allosteric stabilisation”, a real wonder in molecular biology.
Why is your research important for the scientific community?
Understanding the mechanism behind allosteric stabilisation and how it happens at the molecular level is essential as it a central theme in enzyme regulation and complex biomolecular systems. We can now say that this modus operandi is paradigmatic for several toxins. For example, B. anthracis, the causative agent of anthrax, uses a toxin that transitions from disorder to order in a similar fashion, upon binding with a different protein.

Why is your research important for society?
These toxins are responsible for dangerous infections in hospital settings and elsewhere. Inside the cells, they use their active core to impair the immune system and evade its response. The active site has always been the first target for drug developers, but what if we find antidotes to prevent activation of the enzyme by attacking the allosteric site instead? Thus, understanding the mechanisms underlying allosteric regulation in proteins may pave the way for new drug developments due to its versatility in providing desirable selectivity against protein targets while minimizing toxicity and other side effects.
How important is the methodology that you used, cryo-EM, for your discovery?
Cryo-EM was crucial for elucidating how ExoY binds to a filamentous protein like actin. Since filamentous proteins cannot be crystallised, it’s impossible to solve their structure by X-ray crystallography.

