A molecular syringe paves the way to new therapeutic approaches
The attack mechanism by which certain pathogenic bacteria inject deadly enzymes into their hosts
From the advent of Nobel Prize-winning CRISPR gene-editing methods to the introduction of COVID-19 mRNA vaccines, human control over biomolecules has emerged as one of the most significant advancements in science and medicine over the past decade. These new biotechnologies require precise knowledge of existing molecular mechanisms to mimic these processes in a controlled manner. In a collaborative effort, an international team from the University of Geneva (UNIGE), the Max Planck Institute of Molecular Physiology in Dortmund, and Heinrich-Heine University of Düsseldorf has now gained important insights into the mechanism by which certain pathogenic bacteria inject deadly enzymes into their hosts. The detailed molecular understanding of the different steps behind this process suggests potential applications of Tc toxins in biotechnology, such as biomedical devices and biopesticides.
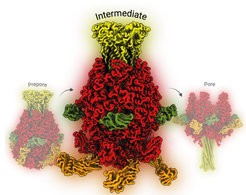
Tc toxins are protein complexes produced by certain bacteria that act like tiny syringes. They attach to the surface of a host cell and, when conditions are right, end up injecting harmful enzymes into the cell. The molecular mechanisms underlying Tc toxin release and intoxication, including toxin activation, receptor binding, and membrane permeation, have been previously reported by the Raunser lab and others. However, detailed insights into the actual injection procedure remain elusive, relying solely on the observation of initial and final states.
The ability to mimic or control these ways of action would enable the development of targeted therapies for bacterial infections, innovative drug delivery systems, and environmentally friendly biopesticides. For example, innovative drug delivery systems aim to improve the precision and effectiveness of treatments, by ensuring that drugs are delivered directly to the target site in the body, for instance cancer cells, minimizing damage to healthy tissues. Nonetheless, the ability to set-up such promising strategies requires detailed understanding of the existing natural process of infection by such toxins.
In recent work by UNIGE, Max Planck Institute of Molecular Physiology and Heinrich-Heine University of Düsseldorf, researchers used a combination of techniques to unveil the detailed mechanism of the syringe-like injection mechanisms of Tc toxins: cryo-electron microscopy, single-molecule fluorescence spectroscopy and electron paramagnetic resonance (EPR) spectroscopy. Dr Svetlana Kucher, senior postdoctoral researcher in the Department of physical chemistry at the UNIGE Faculty of Science, and first author of the study, explains: “This combination allows capturing both individual real-time molecular structures and bulk dynamics, thus giving us an unprecedented image of the Tc toxin infection mechanism”.
Enrica Bordignon, full professor in the Department of physical chemistry and vice-dean of the UNIGE Faculty of Science comments: “It was an exciting collaborative journey that brought us to the conclusion that the intuitive syringe mechanism for the toxin injection is actually much more complex and it includes a multi-state pathway. The interdisciplinary approach used here was key to access the result.”
Stefan Raunser, managing director of the Max Planck Institute of Molecular Physiology, explains: “These extremely precise machineries might sound simple as we easily make and use a syringe to inject materials at the human scale level. However, observing and describing this kind of mechanism at the molecular level is extremely challenging. Knowing the precise sequence will help to design a new generation of biotechnology such as molecular syringes for medicinal applications”.