
Philippe Bastiaens
Director, Systemic Cell Biology
Research Concept
Cognition emerges from the pattern forming activity of biochemical networks that execute cell signaling, and we are generally interested in how the dynamics of biochemical networks generate the spatial organization of signaling systems. Our attention is currently focused on the interdependence of membrane dynamics and early growth factor signal processing in cells. In this context, we investigate how the interaction of receptor tyrosine kinases (RTKs), small GTPases and Src family kinases with membrane systems affects the dynamics of signaling networks and thereby change the cell’s perception of the environment. Based on these studies, we opened new avenues for pharmacological interference with oncogenic signaling in cancer cells. We have also started a new synthetic biology research line in which we reconstitute from biochemical building blocks a trinity of signaling, cytoskeletal dynamics, and membrane shape that interact within a closed-loop causality to give rise to a self-organized morphogenic system.
Current Research
The Interdependence of Vesicular Dynamics and Receptor Tyrosine Kinase Signaling
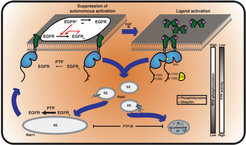
Autocatalytic activation of RTKs coupled to dephosphorylating activity of protein tyrosine phosphatases (PTPs) ensures robust yet diverse responses to extracellular stimuli. The inevitable tradeoff of this plasticity is spontaneous receptor activation and spurious signaling. We could show that a ligand-mediated switch in RTK trafficking together with spatially partitioned PTPs is a uniquely suited solution to suppress spontaneous activation in a continuous safeguard cycle while maintaining the receptor’s capacity to transduce extracellular signals ( Sabet et al. 2015, Baumdick et al. 2015 [1, 2] Figure 1).
These studies made clear that vesicular dynamics and the interaction with spatially organized phosphatases are at the center stage in determining growth factor signaling response.
Lipidated, peripheral signaling hubs move to stay at the plasma membrane
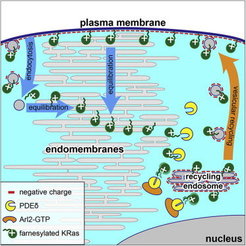
Our laboratory has demonstrated that binding to the GDI-like solubilizing factor (GSF) PDEδ is essential to maintain the subcellular localization and signaling capacity of several farnesylated Ras variants, and that knock down of PDEδ blocks the proliferation of oncogenic Ras containing cells [3]. This work led to the development of new strategies to block oncogenic signaling of Ras by pharmacologically affecting the dynamic cycles that maintain their localization at the plasma membrane [4, 5]. We could show that the capacity for Ras-signaling is inextricably linked to its enrichment at a membrane compartment, which is dynamically maintained by three essential elements – alteration of membrane affinities via lipidation and membrane interaction motifs, trapping on specific membrane compartments coupled with unidirectional vesicular transport to the PM, and regulation of diffusion via interaction with GSFs.
This system constitutes a cycle that primarily corrects for entropic equilibration of lipidated peripheral signaling proteins to all membranes that dilutes their signal transduction capacity (Figure 2). We uncovered how this reaction-diffusion system maintains an out-of-equilibrium localization of Ras GTPases (Schmick et al. 2014, [6, 7]).
Synthetic biology: reconstitution of a self-organized morphogenic membrane
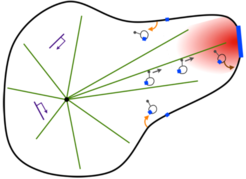
In the context of artificial cell design, we aim to experimentally reconstitute a living membrane system that has the intrinsic ability to translate extracellular information into a morphological response. The basic concept is to generate a stigmergic system [8], in which a trinity of signaling, cytoskeletal dynamics, and membrane shape interact within a closed-loop causality to give rise to an energy-consuming, self-organized morphogenic giant unilamellar vesicle (GUV). This synthetic system mimics the self-organizing process of neurite morphogenesis in response to a growth factor gradient (Figure 3).
Beating the biological uncertainty principle by reversible cryo-arrest
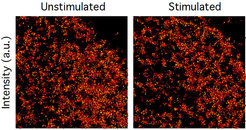
Resolution in fluorescence-microscopy is limited by the time it takes to collect enough photons to obtain sufficient signal-to-noise for image reconstruction. However, molecular patterns in living cells are highly dynamic, which impedes imaging with long acquisition times. We therefore developed reversible cryo-arrest on a microscope by cooling cells to ‑45°C (Masip et al. 2016). We used this to study the micro- and nano-scale organization of RTKs. Consecutive single molecule localization microscopy under reversible cryo-arrest enabled for the first time the observation of EGFR nanoscale cluster reorganization after growth factor stimulus in the same cell (Figure 4).
Literature
1. Baumdick, M., Bruggemann, Y., Schmick, M., Xouri, G., Sabet, O., Davis, L., Chin, J.W., and Bastiaens, P.I. (2015). EGF-dependent re-routing of vesicular recycling switches spontaneous phosphorylation suppression to EGFR signaling. Elife 4.
2. Sabet, O., Stockert, R., Xouri, G., Bruggemann, Y., Stanoev, A., and Bastiaens, P.I. (2015). Ubiquitination switches EphA2 vesicular traffic from a continuous safeguard to a finite signalling mode. Nat Commun 6, 8047.
3. Chandra, A., Grecco, H.E., Pisupati, V., Perera, D., Cassidy, L., Skoulidis, F., Ismail, S.A., Hedberg, C., Hanzal-Bayer, M., Venkitaraman, A.R., et al. (2012). The GDI-like solubilizing factor PDEdelta sustains the spatial organization and signalling of Ras family proteins. Nature cell biology 14, 148-158.
4. Zimmermann, G., Papke, B., Ismail, S., Vartak, N., Chandra, A., Hoffmann, M., Hahn, S.A., Triola, G., Wittinghofer, A., Bastiaens, P.I., et al. (2013). Small molecule inhibition of the KRAS-PDEdelta interaction impairs oncogenic KRAS signalling. Nature 497, 638-642.
5. Papke, B., Murarka, S., Vogel, H.A., Martin-Gago, P., Kovacevic, M., Truxius, D.C., Fansa, E.K., Ismail, S., Zimmermann, G., Heinelt, K., et al. (2016). Identification of pyrazolopyridazinones as PDEdelta inhibitors. Nat Commun 7, 11360.
6. Schmick, M., Kraemer, A., and Bastiaens, P.I. (2015). Ras moves to stay in place. Trends in cell biology 25, 190-197.
7. Schmick, M., Vartak, N., Papke, B., Kovacevic, M., Truxius, D.C., Rossmannek, L., and Bastiaens, P.I. (2014). KRas localizes to the plasma membrane by spatial cycles of solubilization, trapping and vesicular transport. Cell 157, 459-471.
8. Schmick, M., and Bastiaens, P.I. (2014). The interdependence of membrane shape and cellular signal processing. Cell 156, 1132-1138.



