Weak point in pathogenic bacteria
Fight against multi-resistant germs
In the European Union approximately 670,000 people contract infections caused by antibiotic-resistant pathogens every year and approximately 33,000 people die. Despite this enormous and globally increasing danger, only a few new antibiotics have been developed and approved for the market in recent decades. It is expected that only ten new substances will be approved in the next five years. However, most of these substances belong to the known antibiotic classes and act on previously identified weak points of pathogenic bacteria.
Destroying bacteria by a new mechanism of action
In view of the rapidly progressing development of antibiotic resistance, it is of great medical and social interest to find novel antibiotics that kill bacteria in an unprecedented way. A particularly promising target for antibacterial therapies is the protease ClpP. It plays an important role in bacterial metabolism by ensuring the controlled degradation of defective proteins. However, the protease only works in complex with the AAA+ chaperone ClpX. It recognizes proteins that are labelled for degradation, unfolds them while consuming energy and then directs them into the barrel-like degradation chamber of the protease.
A new class of potential antibiotics, the so-called acyldepsipeptidases (ADEP) can initiate the degradation process by ClpP in absence of ClpX. However, this leads to the uncontrolled degradation of essential proteins - with lethal consequences for the bacteria. This unique mechanism of action has considerable innovation potential in the fight against pathogenic bacteria. While all common antibiotics work by inhibiting vital processes, in this new approach an antibacterial effect is achieved by activating a process.
Disarming bacteria
In addition to its role in protein degradation ClpP is also a global regulator in the production of bacterial toxins - termed virulence factors - that are mainly responsible for the disease-causing effect of bacteria. This characteristic also makes ClpP an important target for the development of new antibiotic approaches aimed at the disarmament of bacteria.
The group of Prof. Dr. Stephan Sieber (TU Munich) has been successfully investigating the protease ClpP for years and has already developed a variety of potent inhibitors against ClpP that can stop the production of bacterial toxins. In this context, the group has also made lots of progress in the biochemical and structural characterization of the protease. However, a high-resolution structure of the ClpX-ClpP complex has not yet been resolved.
Elucidating the 3D structure of ClpX-ClpP opens new possibilities
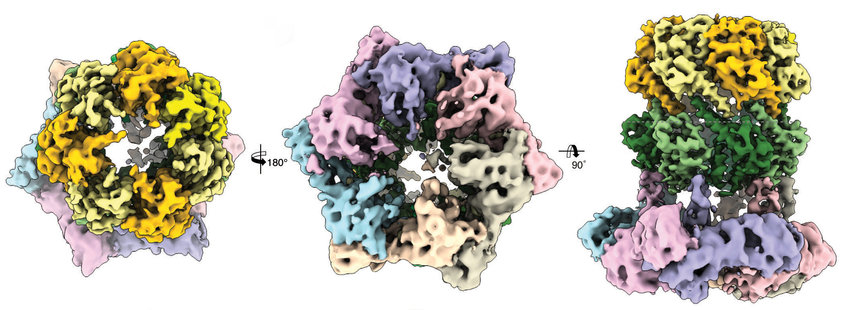
The breakthrough has now been achieved by Dr. Christos Gatsogiannis, member of the group of Prof. Dr. Stefan Raunser. Raunsers team is specialized in the structural elucidation of biomolecules using cryo-electron microscopy. Using this technique, the scientists were not only able to show the difference in the activation mode of ClpP by ADEP and ClpX, but also how exactly ClpP binds ClpX: an atypical asymmetric binding of the six subunits of ClpP to the seven subunits of ClpX.
The structure revealed that the symmetry axes of ClpP and ClpX are not aligned because ClpX is slightly tilted and shifted relative to ClpP. As a result, the channels for the transmission of the unfolded proteins are not coordinated in a straight line. The structural plasticity required for the asymmetric binding of the heptamer ClpX to the hexamer ClpP comes from flexible loops of ClpX, so-called IGF loops. These loops and ADEP bind the same pockets on the surface of the ClpP, surprisingly, with different effects. While binding of ClpX does not lead to a big structural change in ClpP, the binding of ADEP opens the ClpP pore. This leads to an unintended, uncontrolled degradation of even intact proteins without the support of ClpX.
The clarification of this mechanism by the research teams from Dortmund and Munich is a milestone on the way to the development of innovative antibiotic substances targeting ClpP.
JJ/PH

